ELECTRONIC CHARACTERIZATION OF A SERIES OF ORGANIC MOLECULES AND THEIR CHARGE TRANSFER COMPLEXES USING IN SILICO APPROACHES
Keywords:
TTF, TCNQ, Organic material, Molecular mechanics, DFTAbstract
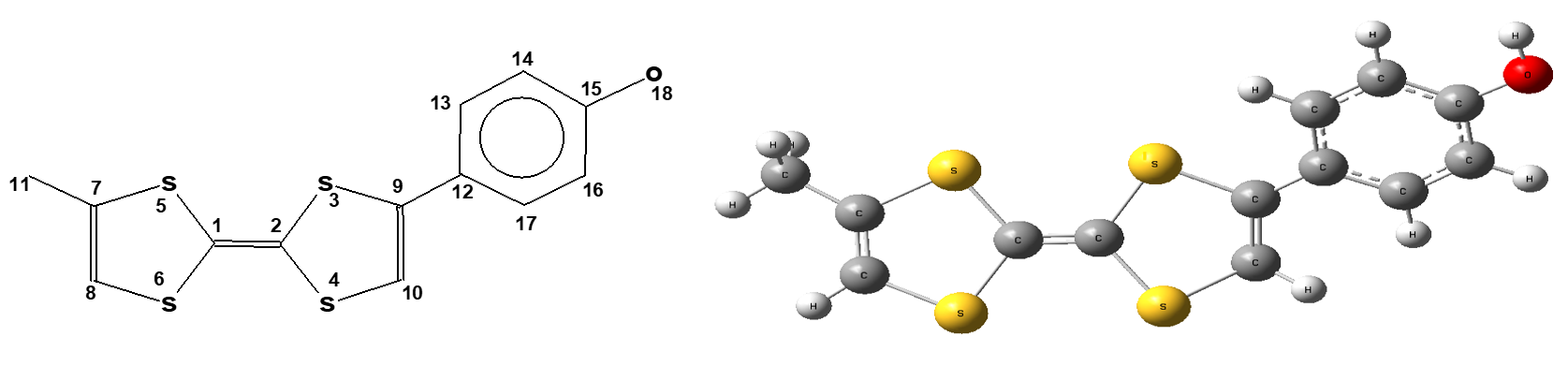
This work explored a series of organic molecules belonging to the tetrathiafulvalene (TTF) family and their charge transfer complexes (TTF-TCNQ), involving asymmetric molecules (donors) complexed with the acceptor molecule tetracyanoquinodimethane (TCNQ). This study was carried out by several in silico methods (molecular mechanics, DFT, PM3 and EHT). A structural study of the donors showed that the majority of the structures of these molecules are pseudo planar and exhibit Cs symmetry. The diagonal angles of the average planes of this series vary from 0.028° to 0.544°, with a calculation error of 0.001°. We found good correlation between the computational values and the experimental values. Charge transfer complexes (CTCs), which have a specific conductivity between 7.6 and 10.6 W -1 cm-1, correspond to a charge transfer between 0.61 and 0.72 e/mol and have a restricted gap of 1.58 to 1.71 eV.
Downloads
References
Wolsky, A.;Giese, R.;Daniels, E. Pour la science, 1989,138-145.
Ferrais ,J.P.; Cowan, D.O.;Walataka,V.V.; Perkstein J.P . Electron transfer in a new highly conducting donor-acceptor complex. J. Am. Chem. Soc. 1973, 95, 948
Andrieux , A.;Bechgaard, K.;DuPoure, C .;Jerome, D.;Acad, C.R.1979, T-288 B-351.
Jerome, D.;Mazaud,A.;Ribaultet, M .;Bechgaard,K. Superconductivity in a synthetic organic conductor (TMTSF)2PF 6.J. Phys. lett.1980, 41, 95-81.
Doublet, M.L.;Gallego-Planas, N.;Philipsen, P.H.T.;Brec,R.;Jobic,S. A new theoretical approach for the electrical properties of TiX 2 (X= S, Se, Te) phases with density functional calculations. J. Chem. Phys.1998,108, 649-656.
Ishibashi, S.;Kohyama, M.Ab initio pseudopotential calculation for TTF-TCNQ and TSeF-TCNQ. Phys. Rev.B.2000,62, 7839.
Abdelmalek, O.;Dibi, A.;Belaidi, S.;Gouasmia, A.;Kaboub, L .Molecular Modeling of New Materials from the Tetrathiafulvalene Family. Asian. J. Chem.2006,18, 1668.
Fabre, J. M.;Gouasmia, A .K.;Giral, L.;Chasseau, D.;Granier,T.;Coulon,C.; Cava, P.Electrical properties and structural data of radical-cation salts and charge-transfer complexes in TTF and TSF families.Synth. Met.1990,35, 57-647.
Leurquin, L.;Ozturk, T.; Pilkington, M.; Wallis, J .D. New precursors for preparing organic conducting materials: synthesis of (R)-hydroxymethylbis (ethylenedithio) tetrathiafulvalene, and the ring expansion of a cyclic sulfate ester. J Chem Soc. Perkin Trans. 1997,1, 3173-3177.
Sing, M.;Claessen, R. T.;Finteis, T.; Hao, S.;Fner,S. H.; Blaha,P.J.Electronic structure of the quasi-one-dimensional organic conductor TTF-TCNQ. Electr. Spectrosc. Relat. Phenom.2001, 14, 717-722.
Zerroug, A.; Belaidi, S.; BenBrahim, I.; Sinha, L.;Chtita, S. Virtual screening in drug-likeness and structure/activity relationship of pyridazine derivatives as Anti-Alzheimer drugs. J. King. Saud. Univ. Sci.2019, 31, 595-601.
Almi, I.; Belaidi, S.; Zerroug, E.; Alloui, M.; Said, R. B.;Linguerri, R.;Hochlaf, M. QSAR investigations and structure-based virtual screening on a series of nitrobenzoxadiazole derivatives targeting human glutathione-S-transferases. J. Mol. Struct.2020, 1211, 128015.
Nour, H.; Daoui, O.;Abchir, O.;Belaidi, S.;Qais,F.A.;Chtita, S.;Belaaouad, S. 2D‐QSAR and molecular docking studies of carbamate derivatives to discover novel potent anti‐butyrylcholinesterase agents for Alzheimer's disease treatment. Bull. Korean. Chem.Soc.2022, 43, 277-292
Ouassaf, M.;Belaidi, S.;Khamouli, S.;Belaid, H.;Chtita, S. Combined 3D-QSAR and molecular docking analysis of thienopyrimidine derivatives as staphylococcus aureus inhibitors. Acta. Chim. Slov. 2021, 68, 289–303.
Ghamri, M.; Harkati, D.; Belaidi, S.; Boudergua, S.; Said, R. B.; Linguerri, R.; Hochlaf, M.Carbazole derivatives containing chalcone analogs targeting topoisomerase II inhibition: First principles characterization and QSAR modeling. Spectrochim. Acta A: Mol. Biomol. Spectrosc.2020, 242, 118724
Khamouli, S.; Belaidi, S.; Bakhouch, M.; Chtita, S.; Hashmi, M.A.;Qais,F.A. QSAR modeling, molecular docking, ADMET prediction and molecular dynamics simulations of some 6-arylquinazolin-4-amine derivatives as DYRK1A inhibitors. J. Mol. Struct.2022, 1258, 132659,
Zerroug, E.; Belaidi, S.; Chtita, S. Artificial neural network‐based quantitative structure–activity relationships model and molecular docking for virtual screening of novel potent acetylcholinesterase inhibitors. J. Chin. Chem. Soc. 2021, 68 ,1379-1399
Kerassa, A.; Belaidi, S.; Harkati, D.; Lanez,T.; Prasad, O.; Sinha, L. Investigations on molecular structure, electronic properties, NLO properties and comparison of drug-likeness of triazolothiadiazole derivatives by quantum methods and QSAR analysis. Rev. Theor. Sci.2016,4, 85-96.
Belaidi, S.; Salah, T.; Melkemi, N.; Tchouar, N. ; Sinha, L., Prasad, O.
Structure activity relationship and quantitative structure-activity relationships modeling of antitrypanosomal activities of alkyldiamine cryptolepine derivatives, J. Comput. Theor. Nanosci.., 2015,12 (9), 2421-2427.
Almi, Z.; Belaidi, S.; Segueni, L. Structural Exploration and Quantitative Structure-Activity Relationships Properties for 1.2. 5-Oxadiazole Derivatives. Rev. Theor. Sci.2015, 3(3),264-272.
Belaidi,S.; Almi, Z.; Bouzidi, D. Electronic structure and physical-chemistry properties relationship for phenothiazine derivatives by quantum chemical calculations. J. Comput.Theor. Nanosci. 2014, 11, 2481-2488.
Belaidi, S.;Mazri, R.;Belaidi,H.;Lanez, T.;Bouzidi, D. Electronic structure and physico-chemical property relationship for thiazole derivatives. Asian. J. Chem, 2013,25, 9241.
Alloui, M.;Belaidi, S.;Othmani, H.;Jaidane, N. E.;Hochlaf,M. Imidazole derivatives as angiotensin II AT1 receptor blockers: Benchmarks, drug-like calculations and quantitative structure-activity relationships modeling. Chem. Phys. Lett..2018, 696, 70-78.
Kerassa, A.;Belaidi, S.;Lanez, T. Computational study of structure-property relationships for 1, 2,4-oxadiazole-5-amine derivatives. Quantum Matter. 2016,5, 45-52.
Khamouli, S.; Belaidi, S.; Ouassaf, M.; Lanez, T .;Belaaouad, S .;Chtita, S. Multi-combined 3D-QSAR, docking molecular and ADMET prediction of 5-azaindazole derivatives as LRRK2 tyrosine kinase inhibitors.J. Biomol. Struct. Dyn.2022, 40 , 1285-1298.,
Belaidi, S.;Belaidi, H.;Bouzidi, D. Computational methods applied in physical-chemistry property relationships of thiophene derivatives. J .Comput. Theor. Nanosci. 2015,12, 1737-1745.
Dermeche, K.; Tchouar, N.; Belaidi, S.; Salah, T. Qualitative Structure-Activity Relationships and 2D-QSAR Modeling of TNF-α Inhibition by Thalidomide Derivatives. J.Bionanosci. 2015,9,395-400.
Belaidi, S.;Youcef,O.; Salah, T.;Lanez, T. In silico approach for conformational analysis, drug-likeness properties and structure-activity relationships of 12-membered macrolides. J .Comput .Theor. Nanosci.2015,12, 4855-4861.
Chermette, H. La modélisation moléculaire : un nouvel outil pour les chimistes. Spectra, 1993, 22, 15-22
Belaidi,S.;Laabassi, M.;Gree, R.; Botrel, A.Analyseemulticonformationnelle des macrolides symétriques de 12 à 28 chaînons basée sur la mécanique moléculaire. ScientificStudy&Research,2003, 4, 27-38.
Belaidi, S.;Lanez,T.;Omari, M.; Botrel, A. Quantitative conformational analysis of dissymmetric macrolides by molecular modeling. Asian. J. Chem.2005,17,859-870.
Allinger, N. L. Conformational analysis. 130. MM2. A hydrocarbon force field utilizing V1 and V2 torsional terms. J. Am. Chem. Soc.1977, 99, 8127-8134.
Allinger, N. L.;Zhou, X.; Bergsma, J. Molecular mechanics parameters. J .Mol .Struct. 1994,312, 69-83.
Kollman , P . Acc.Chem. Res. 1996, 29 ,462-469
Lehn, J. M.;Dietrich, B.; Viout, P. Aspects de la chimie des composés macrocycliques, Editions CNRS, 1995,pp.4-11.
Kaboub, L ; Legros, . J. P. ; Donnadieu, B. ; Gouasmia, A.K. ; Boudiba L. Fabre, ; J.M. J. Mater. Chem. 2004, 14, 351 .
Meislich, H.;Nechamkin,H.;Smarefkin, J. Chimie organique, Editions, McGrawHill, Québec,1979, p.142-144
Belaidi, S.;Laabassi, M.;Gree,R. ; Botrel ,A. Nouvelle approche de la stéréosélectivité dans les macrolides antibiotiques à 20 chaînons par modélisation moléculaire. Rev. Roum. Chim. 2005, 50, 759-765.
Belaidi, S.; Omari, M . Prédiction conformationnelle des macrolides de 13 a 25 chainons par la mécanique moléculaire. J. Alger. Chem.Soc, 2000 ,10, 31-40.
Allinger, N. L.; Cava, M. P.;Dejongh, D .C.; Johnson, C. R.;Lebel A ; Stevens C. L. Organic chemitry, Vol. 1, Editions, McGraw-Hill, New York,1976, 45-47.
Yavorski, B.Detlaf, A. Aide-mémoire de physique,Editions Mir,Moscou, 1980 , pp.416-117;
Benbellat, N.;Le, Gal.Y .;Golhen, S.;Gouasmia, A. K.;Ouaheb, L. Synthesis, characterization and X-ray structures of tetrathiafulvalene-type electron donors, Synth. Met.2012,162, 1789-1797.
Gouasmia, A. K.; Fabre, J. M.;Boudiba, L.;Kaboub, L.;Carcel, C. Synthesis of new unsymmetrically tetraheterofulvalenes-Study of their radical cation salts. Synth. Met. 2001, 120,809-810.
Frisch,M. J, et al, Gaussian 09, Revision, Gaussian, Inc., Wallingford CT, 2009.
HyperChem (Molecular Modeling System) Hypercube, Inc., 1115 NW 4th Street, Gainesville, FL 32601 ; USA, 2008.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Journal of the Algerian Chemical Society

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.

